1) Industrial Production Of Precipitated Silica
> Reaction
Na²Sio³ + H²So4 ------> Na²So4 + Sio²
Sodium Sulphuric Sodium Silica
Silicate Acid Sulphat
+H²O
Water
> Process
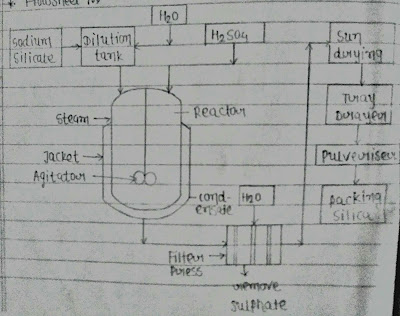
- Precipitated silica is produced by a reation of the sodium silicate & sulphuric acid .
- First of all pure sodium silicate is taken in dilution tank, whare it is diluted by water.
- Now dilution of solution is taken in a reactor in the reactor H²So4 is also added.
- Solution in the reactor is heated by steam a reaction take place between sodium silicate & Sulphuric acid
- Now afrer complition of reaction whole mass is take in filter press to remove sulphate based on compound & give washed of water.
- Tretment mass is drying in sundrying when filter dryed in tray drayer. then pulveriser the mass & packed pure precipitation silica.
> Application
1] Filter softner & performance improvement in rubber & plastic.
2] Cleaning, thickening & polishing agent in toothpaste for oral health care.
3] Food processing & pharmaceutical additive as anti caking, thickening agent, absorbent to make liquid in powders.
4] Food rheology modifier.
5] Defoamer.
2) Industrial Production Of CaCo³
> Reation
CaCl² + Na²Co³ ----> CaCo³ + 2NaCl
> Process
- Calcium carbonate is production by the reation of calcium chloride & sodium carbonate.
- First of all calcium chloride & sodium carbonate both taken in a reation.
- Solution in the reactor heated by the steam reation taken place between CaCl² & Na²Co³.
- The reactor will be attucbed to jacket & also the agitator are mixing the whole mass in reactor.
- the remaining mass will be taken in filter press in the filter press remove of NaCl by a washing of H²O.
- Then the whole mass will be taken of the tray drayer & the pulverised of the product are packed.
> Uses
1 ) The main use of clcium carbonate is in the construction industry material & limestone.
2 ) Calcium carbonate is also used in the prification of iron from are in a blast furace.
3) Industrial Production Of Neoprene Rubber
> Prosecc
- Neoprene rubber is manufactured from the chloroprene
- First of all chloroprene & pyrogallol are taken in stabilizer after it is taken in polymerisation takn here woden pulp, sulphar, NaoH, K²S²O8, & sodium salt of napthalene, sulfonic acid are added.
- Here polymerisation takn place at 40 C in the presence of equal amount of water.
- After complotion of reation whole mass taken in plasticizer here tetra ethyl thiuram di-sulphide is added to increase plasticity & stabilization of polymer.
- Alkali-latex is taken in coagulation tank here coagulation is done is done by acetic acid.
- Now coagulation mass is further taken in brine cooled drum for freezing coagulation & then thin film of the compund is washed by water i have washing tank to remove some impurities, now drayer the mass & 120°C in dry & dyamass is further taken in polymeriser reactor, here alkli mercaptan is added to control the size of polymer.
- Now ploymeric mass is charge in vulcanization unit & valcanization is completed with sulphar in the presence magnesium axide (MgO),zinc oxid (ZnO) as vulcanizing agent cool the mass & cooler & stored as in neoprene rubber.


👌👌👌
ReplyDelete👌👌👌
ReplyDelete🙏🙏
Delete👌👌👌👌👌
ReplyDelete